To see Alexander Nikolaevich Gruzdev, we went to Zvenigorod, where specialists of the Obukhov Institute of Atmospheric Physics of the Russian Academy of Sciences (RAS) are working, making important measurements, analyzing data on the state of the atmosphere, including the ozone layer. This thin layer of ozone protects the plants and all that lives from solar radiation. The scientists noticed as early as 1980s that the increasing impact of the anthropogenic factor in the form of release of chlorine- and fluorine-containing freons had led to a significant thinning of the ozone layer. The famous Montreal Protocol was adopted to address the situation. Have the measures planned then been implemented? Why do ozone holes keep developing? How serious may the consequences of ozone layer weakening be? We asked these and other questions to Alexander Nikolaevich Gruzdev, one of the main Russian specialists in the ozone layer.
Alexander Nikolaevich Gruzdev, Doctor of Physical and Mathematical Sciences, leading research officer of Obukhov Institute of Atmospheric Physics of the Russian Academy of Sciences.
Photo: Nikolay Malakhin / Scientific Russia
— What are the tasks of the Zvenigorod Science Station of the Institute of Atmospheric Physics?
— From the beginning, the station had a broad range of tasks relevant to various branches of atmosphere science. The scientists here studied dynamic processes in the atmosphere, those associated with turbulence in particular, which Alexander Mikhailovich Obukhov once studied vigorously. It was him who formulated the turbulence law, known as the Kholmogorov-Obukhov two-thirds law. There came climate-related tasks: research on aerosol impact, trace gas, cloudiness as a part of the climate system, radiation, etc. These research projects are of interest for both basic and applied science. The most important tasks the station was assigned during its early years, were related to remote sensing, night airglow at the altitudes of 100 km and above.
The station employees continue important measurements, although the range of tasks has narrowed of course. In particular, they measure the levels of nitrogen dioxide and concentrations of ground-level aerosol. Today, the station mainly makes and interprets measurements.
— Let’s talk about the ozone layer. What kind of a part of the atmosphere is it and what are its functions?
— The ozone layer has a very important function: it protects the Earth from the Sun’s UV rays, which are dangerous for living beings. Biologically active radiation can impact the biota and seems to be able to damage living organisms’ DNA. Such radiation is dangerous for people as well. In large amounts, it is bad for the skin, causing dangerous diseases, including skin cancer.
The role of ozone is also that absorbing solar radiation, it transforms the excess energy into heat. Remember that ozone is a radioactive gas involved in the heating of the stratosphere — the atmospheric layer located 10 to 50 km above the ground. The heating affects the atmosphere circulation, which is largely determined by the temperature contrast. Therefore, the uneven distribution of ozone creates the very contrast that leads to changes in the wind situation or circulation of the stratosphere.
The radiation properties of ozone manifest themselves in the troposphere. There, under certain conditions and at certain heights, ozone makes for some greenhouse effect.
It should be mentioned that ozone is a chemically active gas, which is poisonous in high concentrations. High concentration of ozone is dangerous for people as well as for plants, whose activities ozone can suppress. There is a reason why the state of the ground-level ozone is monitored carefully in populated areas. In the capital, for instance, this is the responsibility of MosEcoMonitoring.
Meanwhile, most of the ozone is in the stratosphere. But it should be understood that its share in the atmosphere is very small: millionth shares of the number of air molecules. At that, this layer blocks radiation that may harm living beings on Earth.
However, the distribution of ozone in the atmosphere is most uneven. For instance, there is normally about twice as much ozone at mid and high latitudes as there is in the tropics.
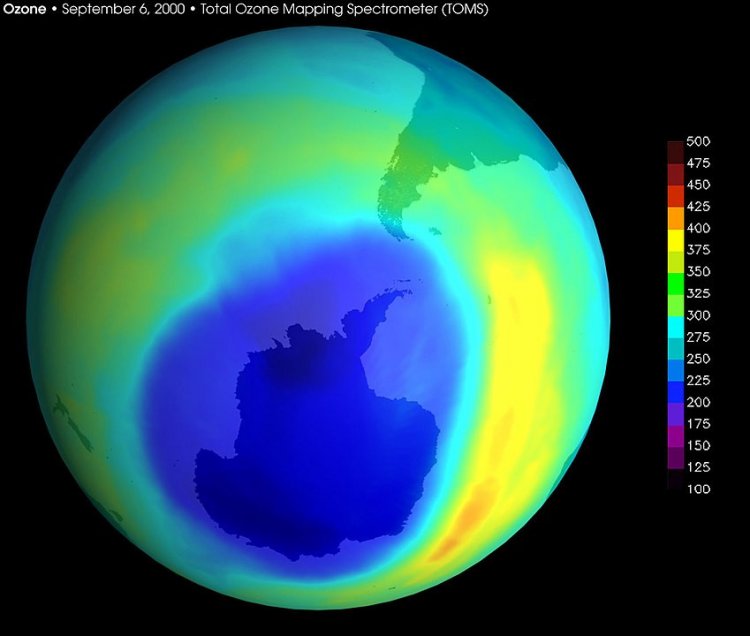
In the 1980s, scientists discovered a significant decrease in the ozone content in the stratosphere in Antarctica in spring — the phenomenon that later became known as the ozone hole. Observations revealed that the ozone content declined considerably during certain periods of time. This has been a relevant issue for many people ever since, one of the reasons being the fact that in the recent decades, similar ozone anomalies were also recorded over the Arctic, in the Northern Hemisphere where more people live and where the phenomenon may have an adverse impact.
— What is known about the causes of such anomalies?
— The cause is twofold. First of all, I would say that ozone is generated by the reaction between atomic oxygen(О) and molecular oxygen (О2). This reaction is most intense over the equator, at the tropical latitudes. The global circulation carries ozone to mid and high latitudes, where it accumulates.
Image of the Antarctic ozone hole, September 2000
— Why the accumulation happens?
— Because, apart from the reaction generating ozone, there are other chemical reactions that destroy it. The rate of destruction largely depends on the height, the time of day, the season, the latitude, and the content of other admixtures. Therefore, the typical time for ozone destruction is distributed extremely unevenly. The destruction rate is much lower in Polar areas, and the time it would take for ozone to deteriorate is longer than it is over the equator. To put it simply, the ozone generated over the equator is gradually carried towards the poles. It has a longer life there. This is why ozone accumulates in those areas. That is, we have some ozone deficit in the stratosphere, and some excess of ozone at mid and polar latitudes.
— Which conditions lead to ozone holes developing then?
— In this case, there is a special photochemical scheme that is activated. The process of ozone depletion is chemical by nature, while the conditions that facilitate the process are dynamic. In this case, the key role belongs to certain admixtures and chemical processes on the surfaces of aerosol particles. Among numerous admixtures that destroy ozone, there are some that destroy it faster and more actively, e.g., chemically active chlorine and bromine radicals. In Antarctic winter, with extremely low temperatures (around -80 degrees Celsius and below) during polar night, polar stratospheric clouds develop some 17 km above the ground. Compounds containing chlorine components are “preserved” on their surfaces. When the polar night is over and the Sun is out, the clouds disappear and the chlorine compounds get into the atmosphere. They break up under the sunlight, generating an excess of active chlorine radicals in the atmosphere, which destroy ozone at those altitudes.
Those are regular phenomena over Antarctica in spring. As I mentioned earlier, an ozone hole developed over the Arctic in some years as well. Although this happens rarer in the Arctic, there were 3 instances over the last two decades when the stratospheric ozone dropped quite significantly. For example, in the spring of 2020, there was a record high ozone depletion over the Arctic, which, however, was smaller both in terms of the area and the degree of ozone destruction compared to the regular phenomena occurring in Antarctica.
— When climate change is discussed, two viewpoints are mentioned: a natural process or an anthropogenic influence as a cause. Is there an anthropogenic factor in ozone holes?
— The current understanding is that there is some anthropogenic impact. In any case, it is responsible for the chemical part of the ozone hole formation mechanism. As we discussed before, the ozone destruction is based on a chemical scheme that involves chlorine. To a significant degree, chlorine in the atmosphere comes from the breakup of compounds produced by people. Those are known freons, chlorofluorocarbons, etc. As regards changes in the atmospheric circulation, whether people have anything to do with them is a big question. Physics is essentially an experimental science. Theories emerge following accumulation of experimental data. In our specialty area, which includes the ozone layer, pure experiments in a natural environment are impossible. The experimental material is observations. It takes measurements over a long period of time to be able to talk about climate change. Unfortunately, we don’t have enough of such data yet. As to the ozone anomalies, fragmentary data from measuring vertical ozone profiles in Antarctica indicate that significant drops in the ozone content in the stratosphere is not a recent development. However, such phenomena are likely to have had a different nature, i.e., they probably were non-chemical.
— What kind of nature, for example?
— A dynamic one, perhaps. But there is no way of saying for sure. The main difference was that the ozone went down in winter rather than in spring like it happens now.
— today have any impact on the occurrence or frequency of such phenomena?
—Of course, they do. The ozone holes develop over the polar areas due to the cooling of the stratosphere. All in all, a negative temperature trend has been discovered in the stratosphere, i.e., the stratosphere temperature has been going down overall for decades. But the problem includes the intensity of the transfer processes as well as temperature. Because the atmospheric circulation carries heat towards the poles as well as admixtures and ozone. Where the circulation becomes less intense, the heat transfer declines, lowering the temperature.
The visual image of the specifics of the circulation is based on stratospheric polar clouds and the concept of a stratospheric polar vortex. In winter, the wind in the stratosphere blows from west to east, kind of encircling the polar area. The wind speed drops closer to the pole. From above, it looks like a vortex around the pole. It is in the stratosphere that the wind peaks. An oval vortex indicates that the polar area inside it is segregated from mid and tropical latitudes. Left to its own devices, it cools down, and the temperature inside it drops. If the vortex has a wave-shaped, disturbed structure, it’s a sign that both heat and ozone get into the area.
That is, an ozone hole developing in spring is connected to the dynamic of the polar vortex. If the vortex is undisturbed, stratospheric cooling is to be expected, as are probably the conditions for the chemical destruction of ozone.
— Essentially, you mean, that they can even be predicted?
— Yes. On the level of models. Of course, some events can be predicted with the modern models, but the advance time is still short, being just weeks.
— Why do ozone holes shrink or expand from time to time?
— The area occupied by this anomaly is characterized as a zone where the total ozone level is below a certain threshold, e.g., lower than 220 Dobson units. This is a conventional boundary. When there was an ozone hole in the Arctic in 2020, the surface area over which the total ozone level was below 220 Dobson units was around 1 million square kilometers. As for Antarctica, the area is 20 times as large, i.e., larger by an order of magnitude. And that with the threshold being 150 Dobson units rather than 220.
Another significant parameter is the anomaly duration. In 2020, the ozone hole over the Arctic was there for a month. Antarctica has an ozone hole over it for months.
When the ozone anomaly area shrinks, that does not mean that the anomaly is “closing.” It is just that ozone-rich air comes to the area. That is why an ozone hole does not have contours in the usual sense.
— How can the anomaly affect the life on Earth?
— The main factor that can be a threat for people is reduced absorption of UV radiation. According to some scientific publications, such consequences have been observed. Moreover, the effect was recorded even in Australia and south Chile. The circulation can cause air with a low ozone level to move north of Antarctica, to populated areas.
about it. We should continue research and take measures the way it was done in the past when measures were taken to reduce the generation and emission of freons.
Besides, there are much more serious problems in the world, social problems in particular, which the atmospheric ozone problem just cannot compare to.
— As regards the Montreal Protocol you mentioned, to what degree has it been implemented?
— There are different views on that in our scientific community. But I would look at its implementation from various angles. For instance, Russia was one of the countries that supported the measures proposed in the protocol. But the implementation of those restrictions in this country took place at the time when our industry degraded for entirely different reasons.
At any rate, the global generation of ozone depleting substances, including freons, has been reduced. And measurements confirmed a significant slowdown in the growth of such compounds in the atmosphere.
However, it is too soon to say that the problem is solved. The thing is that when those international measures were adopted, they targeted specific substances. Therefore, substitutes had to be found. Companies started using other substances as coolants: ones with lower ozone depletion capacity. However, they have other negative properties, including a strong greenhouse effect.
— That is, there is no solution yet?
— Solutions to global problems are adopted by politicians, not by scientists. This is why, by the way, the Intergovernmental Panel on Climate Change was created to inform the public and authorities of possible impacts.
The Diploma of the Nobel Prize Certificate for participation in the Intergovernmental Panel on Climate Change
— Are there any ideas being voiced regarding the ways of intervening into that process?
— This immediately begs the question: Should we intervene? At the moment, we cannot estimate the consequences of such intervention. The technical aspect of the issue is not clear either.
For now, we can only stop generating the substances that have an adverse impact on the ozone layer, and then wait for changes for decades.
— What made you interested in this subject and why did you start studying ozone holes?
— This was something of a natural path in science. I studied at the Atmospheric Physics Subdepartment of the Physics Department of MSU, which was then led by Alexander Mikhailovich Obukhov, the founder of our institute. His successor at the subdepartment was Alexander Khristoforovich Khrgian, whose pupil I consider myself to be. He was one of the main specialists in the atmospheric ozone in the country. In those years, there were high-level specialists and works in this area. By the way, the Russian ozone measuring network was and still is one of the largest in the world.
After my graduation from MSU, I was invited to join the Institute of Atmospheric Physics to continue ozone-related work. And when the publications about the ozone anomaly over Antarctica appeared, I prepared proposals on expedition measurements relevant to the issue and presented my program to the Arctic and Antarctic Research Institute — the leading polar research center.
We performed our first measurements under the Soviet Antarctic Expedition in 1987-1988, and they were received with great interest by international scientific community.
In expedition
I have been to Antarctica just once, but visited three stations: Molodyozhnaya, Mirny, and Vostok. The latter is considered to be the harshest one. Besides, I flew to Vostok and measured ozone concentrations from the airplane. That was quite a process. I needed a permit and everyone's consent. But eventually, the issue was settled by the captain of the crew. He asked what I needed. I said: a 220V power source and a hole in the fuselage. He said: “Oh, we have a lot of holes here — nothing but gaps.” The plane was an IL 14. An excellent plane. This was how ozone concentration was first measured from onboard a plane on the route Myrny – Vostok – Mirny – through a hole in the fuselage.