For years, experts from Tyumen University of Industry (TUI, Tyumen, Russia) have been researching the properties of oxide surface layer. They came up with high-strength structural materials made of aluminum, a metal with superior processability. Surface hardening techniques for aluminum parts developed in-house by TUI researchers promise increased reliability of components and assemblies manufactured at heavy industry and machine production plants
High conductivity, distinctive mechanical properties, low cost and abundance in subsoil make aluminum a promising metal for industrial needs. The unique nature of aluminum alloy surface enables the deposition of a protective coating which is of huge relevance for the production of manufactured parts, assemblies and components of field equipment.
Experts from Tyumen are considering an alternative solution for corrosion-proof and wear-resistant materials and propose an improved aluminum alloy anodizing process utilizing ozone.
Under the leadership of Nikolay Filippovich Kolenchin, the staff of the Functional Coatings Laboratory (founded back in 1980s and relaunched in early 21st century) at the Tyumen University of Industry are performing applied research on the production technology of aluminum-based high-strength materials using ozone-containing solutions, ultrasonic and mechanical vibrations, vibrations and movable cathode tip designs. The laboratory has installed state-of-the-art research and technical equipment enabling complete range of research activities (several types of ozone generators, ultrasonic baths, a vibration unit, a grinding machine for sample preparation, a vacuum pump, a compressor, a distiller, microscopes, multimeters, air treatment system, an oscilloscope, a PMT-3 microhardometer, portable coating hardness/thickness gauges, etc.)
Nikolay Filippovich Kolenchin – Doctor of Technical Sciences, senior researcher at the Center of Advanced Research and a part-time professor of the faculty of material science and structural material technology of the Institute of Industrial Technology and Engineering under the Tyumen University of Industry
Nikolay Filippovich Kolenchin Doctor of Technical Sciences, senior researcher at the Center of Advanced Research and a part-time professor of the faculty of material science and structural material technology of the Institute of Industrial Technology and Engineering under the Tyumen University of Industry (Tyumen, Russia) describes unique benefits of aluminum as a target metal for technologies seeking to improve performance of manufactured parts, explains the difference between the original oxide technology of surface hardening of aluminum alloys and tells which fields of knowledge are updated by TUI researchers’ findings.
“Applied science focuses on the end result allowing one to create products with new properties or improve performance of products in use. The durability of a product depends largely on the state of its surface layer and its ability to withstand aggressive impacts. For most components and mechanisms, increased wear resistance, corrosion resistance are the most important health factors. Specifications for the development of a surface hardening process inevitably call for compliance with environmental regulations as well as product cost and weight reduction.
Aluminum stands out with its possibilities and properties that can be leveraged. Its low density, good workability, non-toxicity, resistance to sparking and its ability to grow a protective film on its surface always draw researchers’ attention. A different matter is every developer’s choice of the way toward their goal. Experimentation is most often centered on transport: aircraft, cars, where most parts are based on aluminum. Some of them need routine repairs, some parts have service lives shorter than the life of the machine and absolutely have to be replaced. In no way does that mean that steel or cast-iron mechanisms are spared. The competitiveness of aluminum vis-à-vis iron-carbon alloys hardly needs to be defended,” said Nikolay Kolenchin.
High-strength materials are based on aluminum because it is one of unique metals.
According to the TUI researcher, “first of all, this is due to its original, natural properties. They include thermal and electric conductivity, low density, processability, non-toxicity. Yet, to a greater extent the metal owes its uniqueness to its ability to adopt new forms. It is so active chemically that its pure form cannot be encountered in nature. Its destiny is to be associated either with oxygen or with water. On the 10-point Mohs hardness scale, aluminum ranks modestly at 2.5-3, but when joined with oxygen, it gets a new name, corundum, and rises to the second place, only inferior to diamond. Aluminum is most abundant among metals in nature: various estimates put its content the Earth’s crust at about 8%. Gray in appearance, it is clad in its oxide which is a gemstone, ruby or sapphire, Al2O3.
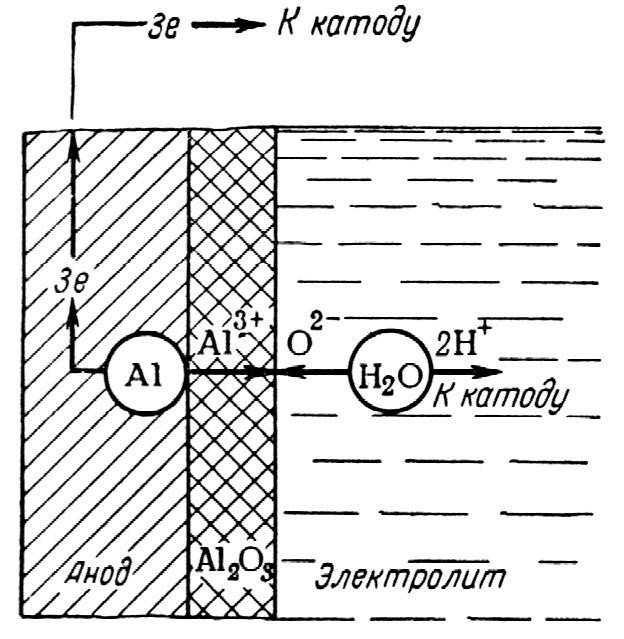
The minute oxide film that covers aluminum is its natural defense that is only adequate to protect it from rain. The first one to sew “thick clothes” for him was our compatriot, professor of Kazan University Nikolay Petrovich Sluginov, and this happened about 150 years ago. When he experimented with aluminum by putting it in sulfuric acid solutions and applying direct currents, the scientist found that its natural oxide film (1-3 nm) began to grow, increased more than thousandfold in thickness. Since then, the world scientific community has made a lot of effort as it refined its methods of achieving the main goal – combining aluminum with oxygen and growing an oxide film on the surface of this metal and its alloys.”
Nikolay Kolenchin explained in detail how such a film is formed:
“Positive pole is connected to aluminum and the negative pole is connected to a flat strip of lead or steel. Oxide, unlike aluminum, is a dielectric, and this fact effectively limits the thickness of the film that grows. Currently all methods of growing this film are limited to a maximum thickness of about 400 μm. Yet, this is enough to ensure that oxide-coated aluminum articles can be wetted with aggressive media, as well as to impart dielectric properties, corrosion resistance and increased wear resistance. There are a lot of ways to achieve the required result. The current passing through the solution may be alternating or pulsed instead of DC. The applied voltage is responsible for ensuring breakdown and can reach 600 V when arc discharge can be seen on the surface a it accumulates oxide. There is a diverse variety of process medium compositions, therefore it would be apt to name only the acids most commonly used for a variety of precursor compositions: sulfuric acid, phosphoric acid, oxalic acid, chromic acid. In addition to acids, process media include alkali.
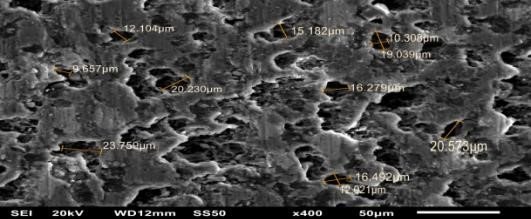
Which parameters of the electrochemical oxidization process are affected by these factors? To find the answer, one has to look inside oxide that forms. There could be two film morphologies: 1) with a clearly oriented structure in the form of hexagonal prisms with a hollow center; 2) having no strict organization.
Cellular organized structure of aluminum oxide
From open sources
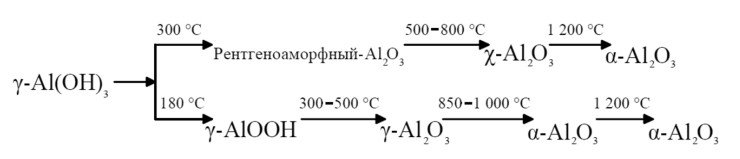
Voltage is a crucial determinant of structure formation. When voltage averages 100V we obtain an organized structure. Successive increases in voltage cause sparks, microdischarges or arc phenomena to appear on the surface as a result of breakdown of the dielectric layer that has already formed. The nature of discharge will determine the depth of the resulting “funnel” where structure is disrupted. Oxide is formed in several modifications, and its phase is determined by the temperature inside pores. The transition of one phase to another can be conventionally represented as follows:
Temperature stages of phase transformations from lower oxides to higher oxides.
(Kalinina A.M. On the relationship between the structure of various alumina compounds and the nature of thermal transformations // Alumina Chemistry and Technology: Proceedings of 4th All-Union Conf. Novosibirsk: Nauka, Siberian Dept., pp. 360-369)
Spark-free anodizing causes oxide formation due to Joule heat released at the bottom of a pore. But this heat is not enough to form a stable phase of α–Аl2О3 or corundum. The temperature determines the crystallization level of the oxide. Therefore, when oxidizing in the micro-arc or arc mode, the temperature in the breakdown channel corresponds to the temperature of X-ray-amorphous oxide transition to crystalline oxide.
The process of interaction of aluminum with oxygen is characterized by two velocity variables: oxide formation rate and oxide dissolution rate. Both are bound by harmonious conformity. This is ensured by electrolyte anions and solution temperature. Low-voltage oxidation requires extensive cooling to reduce the temperature gradient in pores when Joule heat is generated. To that end oxide etching is inhibited by maintaining a process temperature of 0 to 10°С. Another way to control etching rate is by adjusting electrolyte composition.
When high voltages are applied, DC current becomes pulsed and there is no heating of the part. The process is carried out at room temperatures. A prerequisite for interaction between aluminum and oxygen is that both have to be activated. Aluminum is easy to activate. According to various sources, only a quarter of aluminum cations are involved in the reaction with oxygen, and three quarters are released into the solution.
In addition to water, solutions also contain oxygen bound in acidic anions. Still, other scientists are confident that oxygen donors (acid residues) participate in the process of oxidization. There is still no definite answer. What is clear is that neither of agents in this process would arise were it not for activation energy: ionization of water molecules H2O H++OH-; formation of metal hydroxide Al3+ + 3OH- Al (OH)3 and partial or complete dehydration of the resulting hydroxide 2Al (OH)3 2HAlO2 +2H2O Al2O3+H2O».
Aluminum and oxygen ionization process diagram.
What technologies are practiced (or generally recognized) in Russian science for surface hardening of aluminum alloys? What is the novelty of your development?
“In order not to offend all colleagues involved in the development of oxidization technologies, you need to understand the ultimate goal. Today a nanoscale matrix can serve as a membrane or as a form in which nanoscale materials are obtained. Oxide-based dielectric surfaces are also of interest. Anti-corrosion films, wear-resistant surfaces are particular growing methods that imply a specific environment, temperature and electrical conditions, and current sources. The basic has been developed long ago: it’s an electrochemical process. Broadly, there are spark-free processes (at voltages up to 100-150V) and breakdown-based processes (involving spark generation, microdischarges and arc discharges). In no way can the contribution of a particular developer be underappreciated, as all of their efforts are linked together into a single path.
Our contribution is defined by the usage of ozone as an environmentally friendly source of active oxygen in the oxidization process. Without changing the chemical composition of the process medium but rather exciting the medium acoustically and hydraulically, as well as adjusting the shape of the mating electrode, we have been able to obtain oxide coatings with structure, phase composition and morphology different from the classical sort. These changes have improved both the performance properties of the surface (see Fig. 1, change in structure during oxidation in ozone-containing sulphate electrolyte). Along with the nanoscale cellular matrix, large megachannels are formed as a result of ozone’s thermodynamic effect on the growth mechanism of the oxide layer.
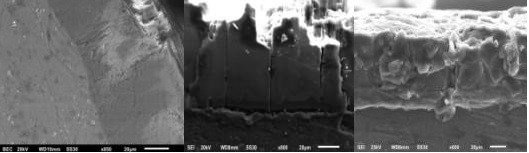
Fig. 1. Oxide film on D16 alloy (95-fold increase) showing large threadlike channels, obtained at a current density of 5 A/dm² and sparging of ozone at a rate of 5 mg/l mixed with air.
N.F. Kolenchin’s studies (photos from the author’s archive)
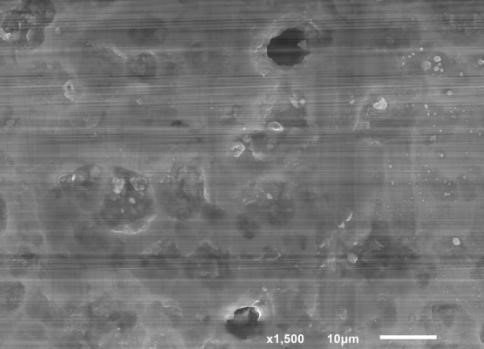
Surface of oxide formed in an ultrasonic field in cavitational burst mode (see Fig. 2). Oxide morphology reveals a cellular nanoscale structure with the formation of wineglass-shaped megapores as a result of thermodynamic reactions in the area adjacent to the electrode.
Fig. 2. Dimensions of caverns on the anode surface generated in an ultrasonic field upon sparging of the electrolyte with ozone and air.
N. F. Kolenchin’s studies (photos from the author’s archive)
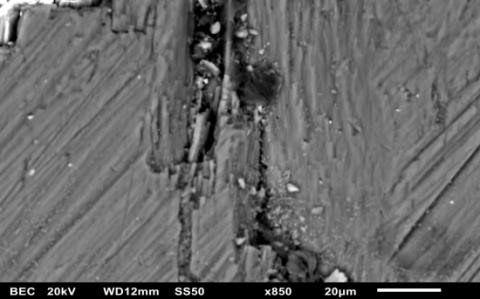
The dynamics of the oxide growth process with anode vibrating at 200 Hz (see Fig. 3). After 15 minutes of the process, film integrity is disturbed. The next 10 minutes are characterized by disintegration in blocks and subsequent overgrowth with the formation of a ridgelike coating structure,” explained the researcher from TUI.
Fig. 3. Fragments of oxide formation exhibiting a particular periodicity with the anode vibrating at 200 Hz.
What is the key difference of your approach in pursuit of improving the oxide formation process?
“If one considers an electrolytic cell with all elements and influencing factors as a single entity, then the change in any parameter will affect other elements of the system: temperature will modify the corrosiveness of the solution; variations in current will be linked to temperature in pores and anion corrosiveness; voltage, while ensuring ion activation, also changes the “oxide skeleton,” etc. We proposed to activate oxygen elsewhere, outside of the electrolytic cell circuit and deliver it toward the electrode ready for interaction. To that end we opted for the simplest electrolyte composition based on sulfuric acid, the simplest of electrical modes and a standard solution temperature. With the help of an ozone generator, we obtained an active portion of oxygen and it delivered to the interaction zone. Ozone is a very strong oxidant; it is an unstable compound that breaks down into atomic oxygen which is no less active than ozone. As a result, we obtained the ideal activation option without changing the enthalpy of the closed circuit of the electrolytic cell. Where is our design a game changer? It affects the oxide formation rate which increased 1.5 times. It modifies film structure as large megachannels appear against the background of hexagonal nanoscale cells. A crystalline oxide phase appeared. That was the first step.
The second step was aimed at increasing the efficiency of the process using an ozone-air mixture. The first step showed that only ozone dissolved in electrolyte is involved in the process. Therefore, it became necessary to increase the solubility of the gas, and for this purpose, an option was proposed to apply an acoustic wave traveling in a liquid in a mode promoting cavitational bursts of minute gas bubbles with large energy releases at the time of the bubble bursting. This increased the solubility of ozone and affected pore shape, the level of film crystallization and, accordingly, the hardness of the resulting oxide. Pore shape turned from cylindrical to the one resembling a wineglass. Such a pore filled with oil is an excellent storage of lubricant in contacting friction parts ensuring superb durability.
The third step was related to alternating motion of the anode or cathode as close as possible to each other. In other words, one of the electrodes vibrated at a certain frequency creating conditions for the occurrence of hydraulic cavitation in the electrode region. The oxide formation mechanism was also altered by the addition of an oxide layer destruction stage with subsequent overgrowth of cracks. The structure obtained a ridgelike morphology akin to fall ice frozen in motion.
Our last solution proposed to improve the process of oxidization in ozone-containing electrolytes was to change the traditional shape of the cathode from flat to needle-like or tipped.
The reason was the theory of energy concentration on the pointed part of electrodes used in scanning force microscopes. What did we get? Without increasing current or voltage we have simply concentrated the charge density on the tip of the electrode and brought it as close as possible to the anode. In order to prevent burning out of the anode material, we used a cathode with a rotating fine-gauge wire tip. Based on the structure formation pattern and phase composition, a conclusion was made about the vector distribution of electron flow density along the moving electrode,” Nikolay Kolenchin explained in detail.
How did you prove the reliability and efficiency of the proposed aluminum alloy anodization technology?
"All investigation and testing techniques were based on standard methods. In addition to laboratory studies, oxide coatings were formed on the surface of real-world parts of oil pumps, gasoline pumps, groundwater pumps and have passed production tests that indicated improved operating performance,” the researcher clarified.
Who are the potential customers of your oxide technology for surface hardening of aluminum alloys? Have the proposed solutions already been field-tested anywhere?
“One of the hardening technologies was deployed in a gasoline pump production shop at a motor-and-tractor electric equipment plant. Industrial-scale application is currently hindered by limited supply of products and maintenance services. It is often unprofitable to set up a site for hardening 10 to 15 parts. The market shapes the demand for services, and now is hardly the time for engine designs capable of surviving a million-kilometer mileage. But I believe that everything will be back to normal quite soon. It boils down to competition between manufacturers,” the researcher from Tyumen commented.
What is your team working on now?
“We endeavored to overcome the thickness barrier, which could upgrade the status of the oxide from a coating to a separately grown structural material. This will be possible when the thickness of the layer exceeds 2 mm. So far, there is a process model and an idea for implementing the experiment. In addition, we have improved the design of the pointed cathode and are creating an experimental version of our setup. These steps will allow us to manage the oxide formation process to create new materials and coatings,” Nikolay Kolenchin said as he explained his plans.
So, the modification technology of Siberian researchers aimed at improving the mechanical and operational performance of the hardened surface layer on aluminum alloy promises an alternative solution to contemporary application problems.
Photo slide