Modern civilization cannot exist without energy. In everyday life, we use convenient electricity, which is transmitted through centralized power grids. But the scientific community regularly reminds us that power grids are not working as efficiently as they could and calls for switching to distributed energy. The main element in such a system is a large storage device that accumulates loads of electrical power and then gives it out as needed – just like the phone battery that is familiar to everyone. Redox flow batteries designed by employees of the D. Mendeleev University of Chemical Technology of Russia use the same principle. What is the advantage of this type of batteries and how are they arranged? Mikhail Petrov tells.
Mikhail Petrov is a Candidate of Physical and Mathematical Sciences, Head of the Scientific and Educational Laboratory “Electroactive Materials and Chemical Sources of Current” of the D. Mendeleev University of Chemical Technology of Russia.
− Mikhail, first of all, tell us about the history of the laboratory and its main tasks
− Formally, its history began in the summer of 2020, but in fact, it appeared much earlier – in 2014. Back then it was a purely scientific laboratory of electroactive materials and electrochemical energy. It was founded by Professor Mikhail Alekseevich Vorotyntsev and Yury Vyacheslavovich Tolmachev. That is why the laboratory covered two areas at once, which its founders were engaged in – electroactive materials and electrochemical energy.
The topic of electroactive materials was more fundamental. The staff investigated materials that can change their properties under the influence of an electric current. The second topic, dedicated to electrochemical energy, was directly related to the development of special flow batteries. I must say that there are many types of flow batteries. But Professor Vorotyntsev and Yury Vyacheslavovich Tolmachev had a revolutionary idea to create a type of battery that no one had made before, and which worked in a completely different way, namely due to an autocatalytic reaction. Such a battery could discharge quickly, giving out a large specific power.
Gradually, the laboratory was joined by new employees, including young scientists and UCTR students. And naturally expanded scientific topics, delving into the development of flow batteries.
In the end, it was decided to reformat the laboratory by including an educational component in it. We have opened a master’s degree program on smart power systems. And now we are not only developing the current direction but also teaching young employees.
− If we talk about energy, which energy storage devices are traditionally used today?
− To begin with, in general, energy storage devices are now being used much more often. Every year, humanity produces and consumes more and more electrical energy. At the same time, the gap between how much energy is produced simultaneously and how much energy is needed at any given time is growing.
It’s hard to imagine that a nuclear power plant would be adjusted to the operation of my kettle or a solar power plant to the operation of a washing machine. Therefore, the energy industry needs energy storage facilities, where surplus is stored, which can be used if necessary. The simplest and most understandable example is lithium-ion batteries in our phones. We can charge them and use the phone for a day or two.
All batteries or accumulators are chemical sources of current in which electricity is stored in the form of chemical energy. Simply put, electricity comes from an external network, triggering electrochemical reactions with certain substances. Electrical energy is converted into chemical energy when charged, and vice versa – chemical energy into electrical energy when discharged. And the operation of the battery is cyclical: discharge-charge.
However, this is not the only way to store electrical energy. There are supercapacitors, thermal machines, and mechanical systems, as well as various exotic options in the form of flywheel systems. And most of the energy in the world is stored in pumped-storage power plants, where it is saved due to the elevation difference.
Despite the variety of energy storage devices, the share of chemical current sources – batteries and accumulators – is constantly growing. And more and more electricity is stored in batteries and accumulators of various types.
Sometimes I see comments on the Internet, “Is it really impossible to make such a battery that would be suitable for everything?” A universal battery that charges quickly and can withstand daily recharging for several years. One that would be suitable for both a phone and electric car, or other devices.
The obvious answer is no. I would like to note that each of the types of batteries – fuel cells, flow batteries, lithium-ion batteries, lead-acid batteries, nickel-cadmium batteries, and others – has its pros, cons, and its own characteristics. You can see the difference thanks to the so-called Ragone plot. The specific power of the battery is deposited along the X-axis, and the specific capacity is deposited along the Y-axis. This graph indicates how much energy a battery weighing one kilogram can store, and how quickly it can release the accumulated energy.
For example, lithium-ion batteries have always been considered the golden mean. They are good in terms of specific power and store a lot of energy per unit of their volume. But there are different batteries, and each of them is suitable for certain applications.
− What is the advantage of flow batteries?
− Redox flow batteries are a kind of hybrid, something in between a battery and a fuel cell. As a rule, such a battery consists of three parts. First, it is the battery itself – a membrane-electrode unit in which all electrochemical reactions occur. Second, two fuel tanks are placed separately from the battery. In any chemical current source, two reactions always occur – in one something is oxidized, and in the other, it is reduced. And there are just two fuels in the flow battery, or, more strictly speaking, electrolytes. They are continuously pumped through the membrane-electrode block, while one electrolyte is oxidized and the other is restored. This is why the battery is called flow.
And the membrane-electrode block of the flow battery resembles a kind of “sandwich” of different layers. One fuel is supplied to one part, and another is supplied to the other. Accordingly, in one part the fuel is oxidized, and in the other, it is reduced. And the battery parts are separated from each other by a semi-permeable membrane that prevents the two electrolytes from fusing.
One of the main advantages of a flow battery is its durability. According to some estimates, the cycle can take place up to 200 thousand times. Therefore, sometimes flow batteries are called eternal.
Of course, this is not the case, because the fuel degrades, as does the battery itself. The leakproofness and the electrode materials on which the reaction takes place eventually deteriorate. But, nevertheless, its resource is quite high compared to many other electrochemical energy storage devices.
The second fundamental advantage is that the fuel tanks and the membrane-electrode unit are mounted separately from each other, that is, in different housings. For example, in a lead-acid battery, all parts are assembled in one housing. Therefore, the capacity is always highly dependent on the desired power.
When working with a flow battery, it is easier to change the parameters for certain tasks. If you need a more capacious battery, it is enough to use more fuel tanks. If power is needed, the membrane-electrode block increases and the dimensions of the fuel tanks remain the same.
But, like any other technology, flow batteries have their drawbacks. One of them is related to the advantage I mentioned earlier. Fuel tanks, which are installed separately from the battery, weigh quite a lot by themselves. Therefore, the specific capacity of a flow battery (an indicator of how many watt-hours of energy one kilogram of a flow battery can store) is significantly lower than that of lithium-ion or other types of batteries. Therefore, it cannot be used in phones or passenger cars. However, since recently there are new projects developed for trucks.
Another obvious feature is related to pumps, which require energy to operate. If the battery is small, it will not generate energy to power its own pumps. If the battery is large, then there will be surpluses when generating energy. Therefore, redox batteries are economically advantageous only on a large scale: for example, when used as a backup power source in factories or a storage device in large power systems. For example, a solar power plant will charge a redox battery during the day, which will give electricity to the external network at night.
− What materials are flow batteries made of?
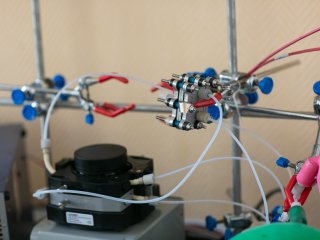
− I’ll start with the battery design. For us, this is the main reason for joy and pride, because we can create prototypes of batteries right in the laboratory. We have a room that we call a fab lab. Various computer-controlled machines are installed there: a milling cutter, laser cutters, laser engravers, as well as a press and lathe. Therefore, we can simply buy the necessary sheet materials and create a variety of batteries directly from them.
Inside the membrane-electrode block, as I have already noted, there are different layers. First, the plates are made of titanium, aluminum, and other metals. They ensure the tightness of the battery. In the very middle of the battery, there is a membrane – a polymer film resembling a stationery file for paper sheets. It lets protons through but resists water and larger ions, uncharged molecules.
Another important component is the electrode materials on which all chemical reactions take place. Most often we use carbon felt or, for example, recently popular carbon paper. It really looks like black paper with a rough surface. It is cut on a laser and a layer is inserted into the battery. It is on this paper that an electrochemical reaction takes place: molecules on the surface are oxidized or reduced. In fact, electrodes and membranes are the key parts of batteries. They are also the most expensive.
By itself, the process of creating one single cell of a flow battery cannot be called complicated. All it takes is to purchase the necessary materials – an electrode, a membrane, plates for flow fields – and assemble a sandwich of about 20 layers, and then connect them with conventional nuts and bolts. The installation is sealed, and the channels filled with liquid are connected to the pump.
Today, about 200 flow batteries have already been installed in the world. They are used in China, Australia, and Germany.
− Are we talking about large installations?
− Correct. We are talking about capacities ranging from tens of kilowatt-hours to tens or even hundreds of megawatt-hours. These are quite large energy storage devices that occupy an area the size of a room or an entire hangar. And, of course, this is not one battery, not one single cell. This is a kind of complex in which many, many single cells of flow batteries are connected.
By the way, China promises to open the largest battery in the world soon. Its design capacity is estimated at 800 megawatt-hours. This is comparable to the energy consumed by 100 apartments of a multi-story building in one year. There are already its first photos showing a huge hangar the size of several football fields.
− Tell us more about fuel for flow batteries.
− Today, fuel from solutions of vanadium salts is mainly used. But I’ll make a reservation right away that there are at least several hundred systems of flowing batteries. Accordingly, different types of fuel and production processes are used. At one time in the laboratory, we started with a hydrogen-bromine battery. Bromine salt is quite affordable because there are a lot of bromine reserves, it is cheap. Therefore, the batteries were inexpensive.
− Batteries are known to explode, oxidize and deform. If we are talking about large capacities, how safe are flow batteries?
− This type of battery is somewhat safer than the others. They are not prone to fires. There are simply no compounds in them that can enter such reactions. A vanadium battery cannot heat up and explode. This is the main advantage in terms of safety.
The only significant danger lies in the possible depressurization of the battery, in which the fuel will come out. It’s not so scary for a vanadium battery – the battery will simply stop working (while vanadium salts in slightly concentrated sulfuric acid solutions will not pollute everything around much), but for some bromine flow battery depressurization threatens with bromine contamination, and this is serious.
− Speaking about the huge battery currently built in China, how long can it work?
− Let me remind you that its design capacity is 800 megawatt-hours. And the battery capacity is 200 megawatts. Relatively speaking, when fully charged, it will work at maximum power for up to 4 hours, and then it will need recharging. How many recharge cycles a flow battery can withstand is a separate story, which is determined by many factors. In general, the best laboratory samples of flow batteries are charged and discharged up to 200 thousand times. But, of course, industrial models degrade faster. However, they will definitely bear about 10 thousand cycles. In general, flow batteries have been operating in the industry for about 10 years.
If we talk about a vanadium battery, it has one significant advantage. Both of its electrolytes are vanadium compounds. If the fuel is mixed, and this actually always happens in all flow batteries or other chemical current sources (because it is impossible to create such a membrane that would be permeable only to what is needed), then nothing terrible will happen. Unless the battery begins to unbalance, when due to the overflow of fuel, one side stores 100 watt-hours, and the other – only 90. To solve this problem, experts use the rebalancing method. Roughly speaking, they drain the fuel, conduct special electrochemical reactions, and get ready fuel again. Therefore, sometimes vanadium fuel is even leased.
− What about ecology? We try to dispose properly even ordinary batteries. How will flow batteries, including units of giant stacks, be disposed of?
− As far as I know, this issue has not yet been raised in the scientific community. Primarily because the created flow batteries are still in service. But in general, there are no heavy toxic metals in their composition. The same vanadium is a metal quite common in the earth’s crust but scattered at the same time. Plus, vanadium fuel does not need to be disposed of, since it can be recharged and put into the process again. Carbon paper in the battery itself also does not require recycling.
Therefore, for now, the issue of environmental friendliness remains open, because we do not touch the deeper production processes of all components of the flow battery.
− Since the direction is quite new, it is clear that there are a number of unresolved problems. Which ones should be solved first?
− I will tell you about our laboratory because the problematic issues are similar in the framework of world practice. Now we are facing three tasks. The first is technological. Single cells of flow batteries with low power of 1–2 watts work quite well. They show good specific power and capacity. But to work in the real world, they need to be scaled: that is, we need to assemble cells into stacks, and combine the stacks into huge systems. Therefore, today the laboratory staff is focused on turning small cells into large stacks while maintaining their high performance and efficiency, and increasing the amount of fuel to maintain efficiency at the same time. The task is extremely utilitarian, but there are many fundamental issues associated with it.
The second task is related precisely to the fundamental directions. Today we are creating different flow batteries: vanadium, hydrogen-bromate, with which everything began, anthraquinone-bromate (anthraquinone is already an organic compound). For hydrogen-bromine batteries, it is necessary to choose the conditions for starting an autocatalytic reaction that accelerates itself. In addition, bromine is a very aggressive environment that leads to the degradation of electrode materials, gaskets, and other things, from which sealing suffers. Therefore, we are now looking for bromine-resistant materials. In some ways, we are reinventing the technologies of the old masters. Because in the Soviet Union, a similar material was known and produced on an industrial scale.
If we talk about anthraquinone-bromate batteries, then there are issues of synthesis. Quinones are substances by which electrons are transmitted inside many living organisms, including us. There are no wires inside a person, but various electrochemical reactions take place here when electrons jump from one molecule to another. Similar structures are used in our batteries. And now, first of all, we are looking for a cheap way to synthesize such organic compounds for batteries that would still be stable during long operation.
And the third task is related to vanadium. The fact is that vanadium is rapidly becoming more expensive. It is commonly used in various alloys, including as an alloying additive to steel. But recently, China adopted a decree on the use of vanadium steel in various spheres, including construction. Accordingly, it is necessary to look for ways to obtain vanadium and vanadium electrolytes. Now, most of the vanadium is extracted from the waste of metallurgical industries. The idea of obtaining this metal from the sludge of the CHP is expressed since oil and fuel oil contain a lot of vanadium.
− The University of Chemical Technology of Russia has always been associated with professional chemists and technologists. However, you act as a physicist here. To you, how important is multidisciplinarity in modern science, when various scientists work in one team?
− In our case, multidisciplinarity is dictated by the scientific tasks. The team should also include chemists who synthesize the necessary substances and develop technologies for producing electrolytes. Nonetheless, we cannot do without electrochemists who understand electrochemical reactions, know what materials to take, aware of conditions and how to optimize them, and so on. There must be engineers who create battery design, who know how to assemble it, what materials to use. And, of course, there should be physicists who, for example, solve problems related to the simulation of flow batteries. After all, you can’t build all battery options. There simply won’t be enough time for this! Therefore, multidisciplinarity is the only way to create an effective system that works well across several areas.