Scientists of the Institute of Biology of the Komi Scientific Center, Ural Branch of the Russian Academy of Sciences (Syktyvkar) conduct experimental genetic studies of aging and longevity. The scientists consider the mechanisms of longevity in combination with a number of factors on model animal organisms and study underlying causes of aging rate. The results analysis will highlight the most promising directions for future developments in slowing down the aging processes in humans.
Understanding the process of human aging and the search for possible ways of achieving longevity is an eternal and controversial topic that still captures the attention of a wide range of specialists. The development of innovations and advanced technologies has changed the living conditions of modern people; thus, it has pushed back the boundaries of manifestations of biological aging, contributing to the conservation of strength and resources, prolonging the full functioning of physiological systems, and even providing an opportunity to reproduce offspring in adulthood. Nevertheless, the irreversible process of aging and the emergence of age-related diseases force scientists to pay attention to a complex of different reasons, which cause changes in the biological component of the human body. The study of regenerative processes, gene expression, pharmacological correction methods will make it possible to understand the cause and, presumably, to slow down the pathological transformation of tissues and, therefore, increase the number of long-livers.
The employees of the Institute of Biology, Komi Scientific Center, Ural Branch of RAS (Syktyvkar) are investigating the factors that influence lifespan, age-related changes in immunity, activities, and the body’s antiviral and antibacterial responses. Experimental investigations help explore some key molecular mechanisms of anti-aging effects related to stress response, antioxidant and anti-inflammatory cell protection; in particular, they help understand the potential of genes responsible for longevity regulation in different mammalian species and the patterns observed in them.
Aleksey Aleksandrovich Moskalev, Professor, Doctor of Biological Sciences, Corresponding Member of RAS, Head of the Laboratory of Geroprotective and Radioprotective Technologies at the Institute of Biology, Komi Scientific Center, Ural RAS Branch (Syktyvkar), Chief Scientific Associate of the Scientific and Clinical Gerontology Research Center (Moscow)
Aleksey Aleksandrovich Moskalev. professor, Doctor of Biological Sciences, Corresponding Member of RAS, Head of the Laboratory of Geroprotective and Radioprotective Technologies at the Institute of Biology of the Komi Science Center, Ural RAS Branch (Syktyvkar), Chief Researcher of the Scientific and Clinical Gerontology Research Center (Moscow), talked about different research aspects and results achieved regarding the longevity issue.
“The genetics of longevity can be studied in long-living people (people over the age of 90-100). This is the most difficult pathway for several reasons. First, there are quite few long-livers, and even fewer of those have had their genome (hereditary information of chromosomes) and transcriptome (a set of matrix RNAs reflecting gene activity) sequenced individually. This fact has a major impact on the statistical significance of conclusions and the probability of error. In addition, such scientific studies require a control sample, which is not easy to set up in the case of long-livers. Who would comprise the control? People from the same population and of the same sex? Elderly people of the 80+ age group? What if they also become long-livers (90+) someday? The second genetic approach to aging is model studies with laboratory animals, in which the genes of interest have been activated or turned off by genetic engineering, resulting in significant life extension in such animal lines compared to parental lines. The third source of longevity data is long-lived mammals being the animals most evolutionarily close to humans. This is an experiment that was set up by nature itself. For example, elephants and gray whales live on average as long as most humans, and bowhead whales live about twice as long. There are also mammals that are extreme long-livers compared to closely related species. For example, the naked mole rat can live more than 30 years in the lab, while other lab rodents live 3-4 years (mice, rats). Or small bats, in particular the Brandt's nightshade, which is the smallest long-lived mammal, live up to 40 or more years in the wild with an average body weight of 8 g. By the way, together with my colleague from Harvard Medical School Vadim Gladyshev and the staff of the Beijing Genome Institute, we decoded its genome and liver, kidney and brain transcriptomes for the first time in 2012. However, we already know how to live for 30-40 years.
There are even more extreme long-lived species, such as the Greenland shark (400+ years), the Icelandic cyprina mollusk (500+ years), and the longevity pine (10,000 years). It is possible to compare their genome with closely related “short-lived” species. It is interesting to “spy” on longevity mechanisms in such species and try to apply them to humans. But how? As for longevity, the genes affecting it could be of interest as intervention targets. In general, any drug is an activator or inhibitor of some proteins or the genes encoding them. Once we know which genes need to be activated/suppressed for longevity, we can create new drugs against age-related diseases or slow down the aging itself in humans,” Aleksey Moskalev explained in detail the importance of research on the molecular mechanisms of longevity and age-dependent changes in gene expression.
“At present we have methods that allow us to activate or deactivate certain genes in humans, because many genes, including those related to longevity, can't be regulated by drugs. Genotherapeutic interventions are now successfully used to treat rare inherited diseases, as well as to create a new generation of vaccines against the coronavirus. We may expect the creation of longevity vaccines when longevity-associated gene variants are delivered to cells instead of the “harmful” gene of the coronavirus protein.
“Finally, knowing the genetic differences of long-livers, or people at high risk of age-related diseases (Alzheimer's disease, stroke, heart attack, type 2 diabetes, cancer), the benefits and risks of genome editing technologies, such as Crisp-Cas9, can be investigated in the future. If a patient's genome is sequenced and studied beforehand, editing technologies would make it possible to “match” this genome with the genomes of long-lived patients who are rarely ill. In addition, understanding the dynamics of gene activity with age can contribute to the development of diagnostic platforms for determining biological age, a tool for assessing whether a person's health corresponds to his or her age norm. This would allow us to track the effectiveness of pharmacological and other aging interventions. For example, it would make it possible to establish whether a drug can reduce biological age (aging rate) by comparing the transcriptomic, proteomic or epigenomic biological age (all of them reflect gene activity in one way or another) in a patient before and after therapy.”
Specialists of the Institute of Biology of the Komi Scientific Center at the Urals Branch of the Russian Academy of Sciences are conducting research on various aspects of longevity. Previously, scientists examined the anti-aging properties of black chokeberry and cloudberry fruit extracts, because the quality and composition of food – especially some plant pigments – have an undoubted influence on health.
“At some point we became interested in the potential geroprotective properties of substances from the group of terpenes (in particular, carotenoids) and polyphenols (e.g., anthocyanins), which are abundant in various berries. We knew that these compounds reduce risk markers of age-related diseases in humans (blood pressure, atherosclerosis), increase stress-resistance of cells and meet some other geroprotector criteria. We had to check their effect on the main criterion – increase of longevity. Adding chokeberry and cloudberry extracts to the diet of model animals led to a certain increase in longevity, but I would not call them pronounced geroprotectors. Apparently, they have little effect on the fundamental mechanisms of aging and longevity,” noted Aleksey Moskalev.
Next, the scientists studied the antioxidant activity of fucoxanthin (derived from brown algae) in human cells, suggesting that fucoxanthin may have therapeutic potential to treat age-related diseases.
“Apart from extracts, we also study chemically pure substances. This approach is more productive, because it increases the reproducibility of the results and allows for a more thorough study of dose-response relationships. Fucoxanthin activates the gene of the transcription factor NRF-2, which triggers a whole cascade of genes that determine the body's own antioxidant and detoxifying activity of cells. We were able to show that fucoxanthin prolongs life in two model organisms – Drosophila melanogaster (Drosophila fly) and Caenorhabditis elegans (roundworm), probably the most favorite animals in gerontological research. It also improved the stress tolerance of these animals (i.e., survival under various adverse influences) and the activation of genes that are known to be associated with longevity. Finally, we showed that fucoxanthin slows the aging of human cells in culture,” clarified the scientist.
In most studies, the specialists of the Komi Scientific Center of the Urals Branch of the Russian Academy of Sciences used the Drosophila melanogaster model to identify the key molecular mechanism of age-related changes.
Aleksey Moskalev gave reasons for the scientists’ choice: “Each model system has its advantages and disadvantages. Drosophila are quite distant from humans evolutionarily. They have about half as many protein-coding genes as we do. However, 75% of the genes associated with human diseases have direct functional homologues (genes with a common evolutionary origin) in Drosophila. Thanks to this very model, chromosomal theory of heredity, mutagenesis, animal and human embryonic development genes (homeosis genes, Notch, Wnt, “sonic hedgehog” morphogenes), genes of innate immunity against fungi, viruses and bacteria (TLR), “heat shock” protein genes, human behavior and aging were discovered. These studies have won several Nobel Prizes. By the way, it was Drosophila that was awarded the first fully sequenced genome among animals, which made it possible to develop some precise methods of creating transgenic animals, switching on and off certain genes at will in the desired tissue and stage of development. All the longevity gene groups known to us (mTor, FOXO, IGF, AMPK, NRF-2, SIRT, PI3K) are present in both flies and humans. However, we are interested in finding new longevity genes. We have developed inclusion-exclusion techniques for the Drosophila model of genes of interest, using which we look for long-lived lines and compare their genomes and metabolomes with less long-lived parental lines.”
Thus, the Drosophila model helped us determine the effect of the Aβ peptide on aging, lifespan, and motor activity. According to Aleksey Moskalev, “peptide Aβ is thought to accumulate in tissues, forming plaques and contributing to age-related neurodegeneration. There is another point of view that as a person ages, the barrier between blood flow and brain tissue breaks down and infectious agents such as viruses and bacteria begin to enter the brain, causing inflammatory damage, while the Aβ peptide is an antimicrobial peptide, which is not the cause of neurodegeneration, but rather a marker of it. Drosophila’s life is too short to accumulate its own amyloids in its tissues and suffer from neurodegeneration. However, its gut barrier function deteriorates with age, causing microbes to enter the body en masse leading to the animal's death. We suggested that if the Aβ peptide had antimicrobial activity, it would protect Drosophila from this age-related mortality factor and increase longevity and possibly immunity. However, we did not detect a significant extension of life, nor did we observe an increase in immunity.”
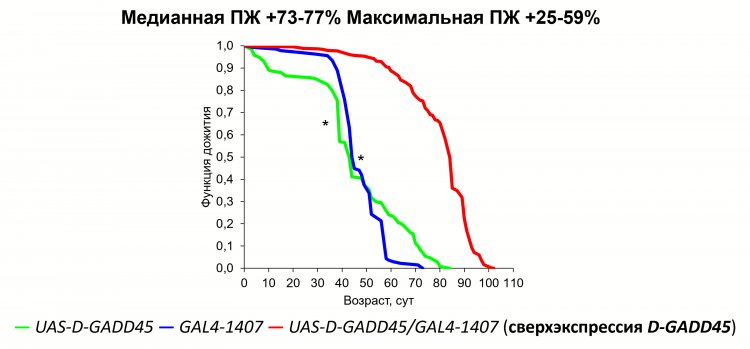
Image 1. Effect of D-GADD45 gene overexpression in the nervous system on Drosophila lifespan
Besides, we studied the effects of genetic and pharmacological interference with H2S metabolism, which accompanies the body aging process.
“Many people remember that a low-calorie diet or periodic starvation can prolong the life of experimental animals. However, few people know that this is associated with the production of hydrogen sulfide by cells deprived of the amino acid methionine from food. Hydrogen sulfide, along with another gas hormone, nitric oxide, plays a signaling and protective role for cells in very low doses. This is why this gas is so important in microdoses, in other concentrations it turns into a strong poison. Hormones have positive effects only in very low doses. The ability of tissues to form micro doses of hydrogen sulfide decreases with aging. We used genetic and pharmacological interventions to increase its production, which had a positive effect on the Drosophila longevity,” reported the scientist.
The unique characteristics of magnetite open up a wide range of its applications from diagnostics to therapeutic agents. Thus, we studied the effect of Fe3O4 nanoparticles on the expression of genes responsible for lifespan in Drosophila.
According to Aleksey Moskalev, “we are seeing a real boom in the search for nanoparticle applications in chemistry. They can be potentially useful not only in engineering, but also in medicine. Our colleagues from the Institute of Chemistry at the Komi Science Center, Ural Branch of the Russian Academy of Sciences (Syktyvkar) are engaged in applied developments in fullerenes, iron oxide nanoparticles, and other nanoparticles. We help them test their safety. For example, our laboratory has shown that Fe3O4 nanoparticles are low-toxic.”
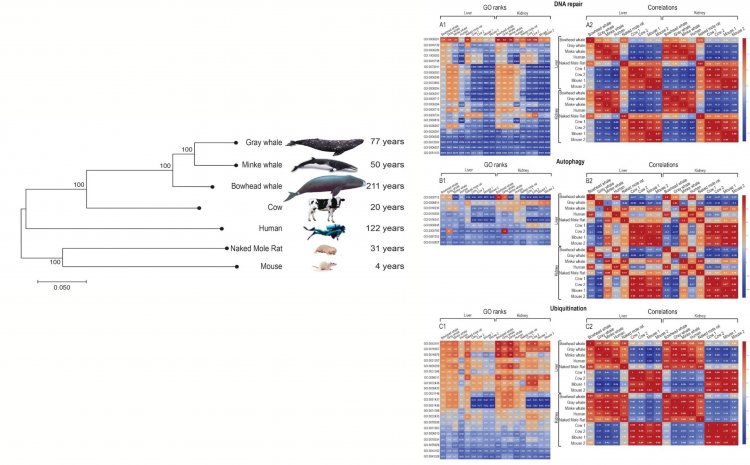
In 2019, together with researchers from the Engelhardt Institute of Molecular Biology (Moscow) and foreign colleagues from Romania (Romanian Academy, Bucharest,) and Israel (Ben-Gurion University of the Negev, Be'er Sheva), the genomes and transcriptomes of long-lived (whales) and short-lived mammalian species were compared. They developed an original method for analyzing gene expression, based on ranking, which helped explain the phenomenon of the longevity of whales, whose organisms are most adapted to stress and cancer (read more in the article “GRAY WHALE TRANSCRIPTOME REVEALS LONGEVITY ADAPTATIONS ASSOCIATED WITH DNA REPAIR AND UBIQUITINATION” //AGING CELL. 2020;19:e13158. //https://doi.org/10.1111/acel.13158).
“As far as I know, this is the first work that compared the activity levels of homologous genes of different mammalian species. The most difficult thing was to create algorithms for “normalizing” gene activity levels (transcriptomes); it is impossible to compare the individuals of different species from different studies without it. As a result, it became possible to see the activity “patterns” of groups of genes performing different functions, typical for long-lived and short-lived mammalian species. The most significant differences were observed in the genes of whales associated with DNA repair, genes of ubiquitination, immunity, and apoptosis. The fact is that somatic cells only have two copies of each of the chromosomes (DNA strands). Therefore, the breakage of any copy causes serious consequences for the cell viability or poses a risk of tumor transformation. An organism will not become long-lived if it is unable to repair DNA quickly and efficiently. Proteins are the cell’s basic building blocks and the molecular machines for almost all metabolic processes. If proteins are damaged (glycated, oxidized), they “pile up” (aggregate) and interfere with the life processes of the cell. The cell has mechanisms for “scattering” and utilization of damaged and redundant proteins called ubiquitination.
“Whales had higher levels of ubiquitination gene activity than other mammals we compared. If a cell is “aged,” it begins to communicate its disadvantage to other cells in the form of signals of chronic inflammation, thereby trying to draw the “fire” of the immune system, which should remove these cells. However, the immune response decreases with age, so old cells accumulate. Usually damaged cells go into apoptosis (altruistic suicide), but senescent cells are resistant to apoptosis. Whales, on the other hand, have higher apoptosis gene activity. Their immune system genes are also more active. Coronavirus has shown once again that we are not likely to live long without a strong immune system,” informed the scientist.
Image 2. Comparison of gene activity in long- and short-lived mammals
According to Aleksey Moskalev, the main preliminary results of research on aging and longevity are as follows: “We have achieved the greatest success in prolonging life in studies on laboratory animals through genetic interventions, i.e., by activating or deactivating certain genes. For example, activation of an extra copy of the DNA damage response gene (GADD45) in nerve tissue made it possible to prolong Drosophila life by 70%. So far, pharmacological effects have had a rather weak effect. My and my colleagues' comparative studies of genomes and transcriptomes of long-lived mammals, such as Brandt's nightshade and whales, have allowed us to find new targets for life extension related to DNA repair.”
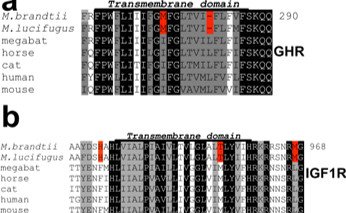
Image 3. Replacements and deletions in the growth hormone and IGF1 receptor genes of Brandt's nightshade
“We also plan to continue looking for geroprotective properties of substances and plant extracts. However, more and more evidence suggest that the key causes of aging do not lie in the cells, but in the stiffness of the extracellular matrix, which is something we plan to dedicate several further experimental works to. Moreover, several of our studies in collaboration with colleagues have made it possible to create models of human biological age – Artery Index and Aging.AI. We have launched two clinical studies using biological age under the influence of biologically active drugs and plasmapheresis,” said Aleksey Moskalev.
So, the studies of the phenomenon of aging at the organismal, tissue, cellular, molecular, and genetic levels are the next step of an active scientific search for possible approaches to slowing down aging and achieving a healthy longevity.